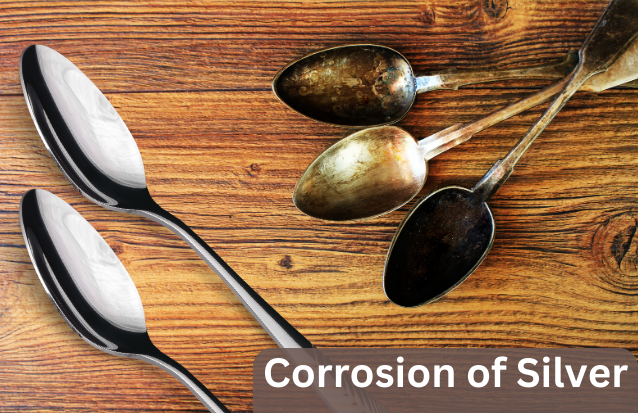
How Will You Recognize the Corrosion of Silver?
Silver is a valuable metal and a favorite among many for its glistening shine. But, in the long run, it is also prone to corrosion. The corrosion of silver takes place when it reacts with certain types of chemicals present in the environment. A black-colored layer of silver sulfide (Ag2S) envelops the silver's surface; this is How Will You Recognize the Corrosion of Silver. The sulfide that corrodes silver is a result of silver reacting with sulfur.
To preserve the good looks and value, it is very important to recognize the symptoms of silver corrosion. The original look of silver can be regained when the coating of silver sulfide is taken out. To maintain the property and integrity of silver, it is always advised to consult with an expert.
Corrosion of Silver
When silver gradually deteriorates because of chemical reactions in the environment it is termed as silver corrosion. Silver is reasonably a stable metal, but it gets corroded when it comes in contact with certain compounds that contain sulfur. Silver, when it gets corroded, loses its natural shine as a dark layer is formed upon its surface. The tarnishing or discoloration further leads to structural damage.
Corrosion of silver occurs when silver sulfide (Ag2S) is formed. It happens when silver reacts with a widespread atmospheric pollutant namely hydrogen sulfide (H2S). Sometimes silver gets corroded when it comes in contact with rubber and some particular kinds of paper.
The chemical formula for silver corrosion is: 2Ag(s) + H 2 S (g) Ag 2 S(s) + H 2 (g).
Signs to Recognize the Corrosion of Silver
1. Change of Color: The most visible sign of silver corrosion is the change of color. When silver becomes dark, it indicates that sulfur-containing compounds have overrun the surface of silver. The black layer containing silver sulfide gives the silver a tarnished appearance. When pure silver is stained, it develops a yellowish shade. A bluish-green or green tinge indicates silver alloys getting corrosion.
2. Loss of Shine: The much-admired shine of silver fades away as it gets corroded. The natural reflectivity of silver becomes murky. The tarnished silver appears lifeless and dull as it loses its signature shine. Silver losing its shine is an obvious sign of corrosion that damages its surface.
3. Irregularity of Surface: The structure of silver can break down owing to chemical reactions. This creates surface irregularity which further develops cracks and pits during the later stages of corrosion. When silver corrodes, its polished and smooth surface becomes rough, and the texture of the metal gets altered.
4. Emission of Odor: At times, the corroded silver releases a sulfurous odor. Although the odor is weak it is quite unpleasant. You can smell the stench when tarnished silver items get cleaned. This odor is a result of silver sulfide or the black tarnish that forms on the surface of the silver.
5. Visible Stains: The surface of a silver item will have spots or stains which can be noticed easily. The stains may be of black or brown color. When such colored marks appear on silver, it indicates that it has suffered some chemical change.
How Can We Prevent Corrosion of Silver?
Taking proper care is very essential to prevent corrosion of silver. You need to keep the silver polished and store it in a proper way. We can take certain measures to prevent the corrosion of silver reactions. They are as such -
- Proper Storage - We need to store our silver items in containers that are airtight. This will ensure no contact with moisture, air or any sulfur-containing compounds. Keeping it away from direct sunlight is also commendable.
- Regular Cleaning - Silver jewelry or other items can be polished on a regular basis to maintain its shine. To polish silver, we need to use a gentle cleaning solution and a soft cloth. This is useful in getting rid of tarnishes and avoids further corrosion.
- Protective Coatings - We can apply certain protective coatings to check corrosion of silver. Lacquer and wax work as an efficient coating. They create a barricade in between the surface of silver and the environment.
However, we must take special precaution not to use harsh chemicals. Some chemicals speed up the process of corrosion when they come in contact with silver. So, avoiding chemicals like ammonia or bleach can do well to the silver.
Conclusion
By understanding the causes and prevention of silver corrosion, individuals can take measures to preserve the beauty and value of their silver belongings. Keeping the silver polished is one the easiest thing one can do to prevent silver corrosion. Silver items are of timeless beauty and it requires some proactive measures to ensure that they remain so. Being vigilant is also important to prevent corroding of silver.
The answer of question How Will You Recognize the Corrosion of Silver is - Silver corrosion can identified by tarnishing, discoloration, pitting, loss of luster, and the presence of powdery or flaky corrosion products. Chemical tests, like reactions with silver polish or mild acids, can confirm corrosion. Preventive measures include regular cleaning, Proper Storage, Regular Cleaning and Protective Coatings .
We need to know the reasons for silver corrosion and then be able to identify it. Then only we would be able to take the correct precautionary steps to check it. Like any other prized possession, Silver also needs to be kept in ideal conditions. This will prevent corrosive elements from attacking silver and damage it.

